Application Techniques for Diamond-like Coatings
What is diamond-like carbon coating?
There are several naturally occurring allotropic forms of carbon. Currently, two of them are the most commonly used in terms of technical applications: graphite and diamond. The differences between these two forms lie in different chemical bonds between carbon atoms in these two structures. Carbon atoms in graphite have strong chemical bonds within each layer (in layer plane) and weak bonds between the layers (planes) (SP2). As a result you have a soft, conductive, transparent, gray material with low coefficient of friction. In diamond crystals all carbon atoms have strong chemical bonds (SP3) in all directions. Therefore, a diamond is a transparent material with the highest hardness value and electrical insulating properties.
Diamond DCL Graphite
Diamond-like bonds SP3 SP3+ SP2 Composition С-Н Composition С
Graphite-like bonds SP2
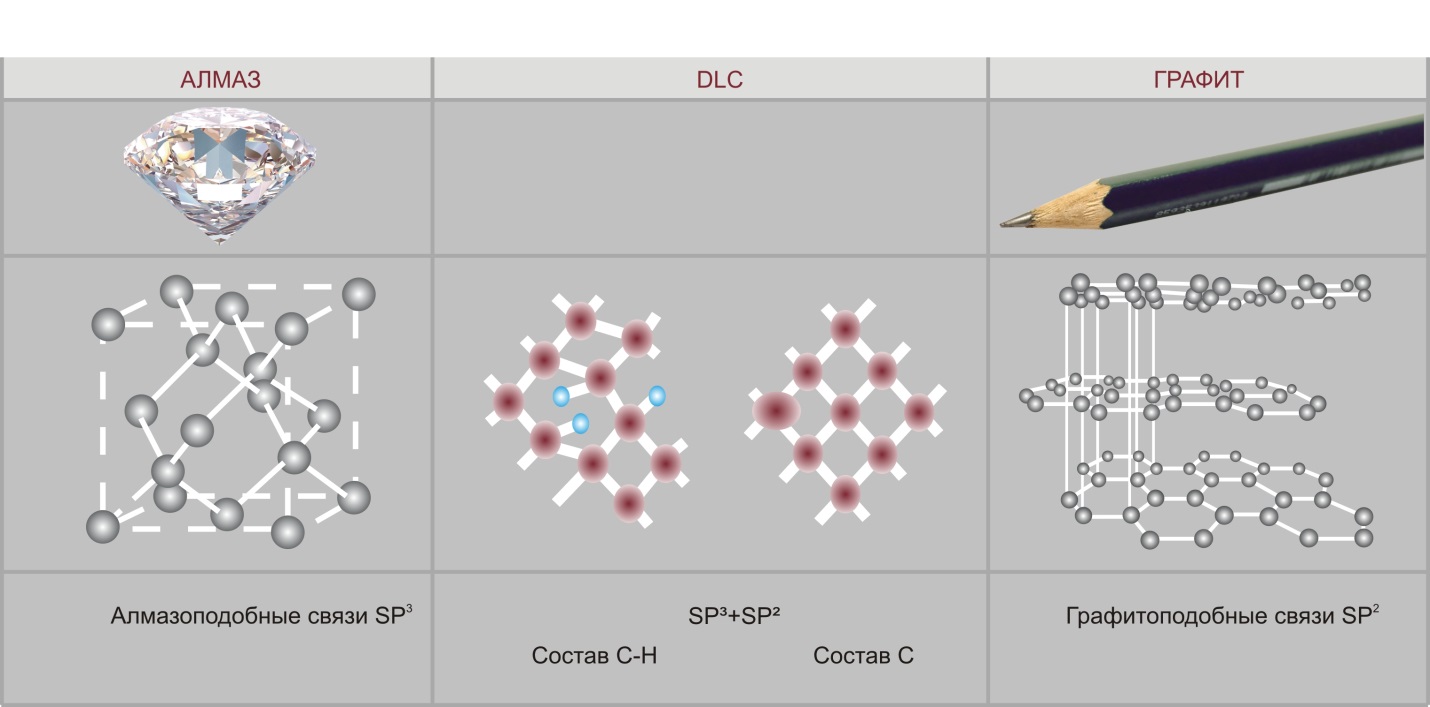
Diamond-like coatings consist of carbon atoms with both types of bonds — diamond-like and graphite-like, that greatly increases the service life of products with such coatings. These amorphous coatings, that have the hardness of a diamond and the friction coefficient of graphite, can be applied within a wide range of temperatures, even at room temperature, to various materials, including metals, ceramics, glass, and plastics.
Diamond-like coating deposition technology, developed by the staff of Special Technologies LLC has several advantages that facilitate its use for mass production. These advantages include ecological cleanliness, firm adhesion of the film to the substrate, high hardness value of the coating which is comparable to that of diamond, and excellent durability. A multilayer coating of complex composition withstands mechanical loading encountered during operating life, providing very good adhesion to the treated surfaces of various forms. These properties are of particular importance for high-alloyed steels used for the manufacture of instruments and tools.

